

Morepen is one of the most vertically integrated companies with sharp focus on building efficiencies in manufacturing quality APIs. Achieving significant share in global and domestic markets, strengthening our product portfolio, creating new customers in advanced markets like the US, Europe and Japan and cementing newer partnerships in emerging markets like China, Taiwan, Mexico, Korea and Russia have been some of our key achievements in recent years.

















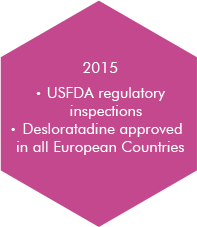



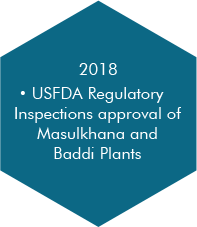

















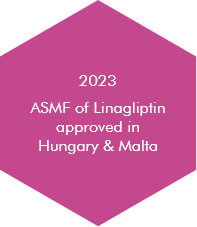


At Morepen, it is our tireless endeavour to offer high-quality generic drugs at most competitive costs by integrating our experience, skills and technologically superior operations.


In order to facilitate our customers to launch new products, we are taking a step forward to prepare dossiers in eCTD format for the finished dosages of the selected products.
This ensures better long term relationship with the customers and also seamless supply of material and documentation at the time of product launch by the customers.


With over 38 active process patents and over twenty block buster APIs being manufactured under cGMP guidelines at Baddi and Masulkhana, we stand as a largest manufacturers of Loratadine and Montelukast Sodium API in the global generic market.


Over 25 new molecules in advance stage of development with non-infringing process, excellent impurity profile and complete documentation. Focus remains remains on new molecules with patent expiry from 3 to 10 years.
In the Diabetic segment company has encompassed the full range of gliptins and gliflozins and has a promising pipeline.
Note: The above products are not for sale where the patents are applicable & still valid

Parwanoo, Himachal Pradesh
India
1000 MT/ANNUM
Production Capacity

Spread over 7 Acers of area with modern and global standard manufacturing units.
US FDA, WHO GMP Approved with
Award winning R&D center
Himachal Pradesh, India
1500 MT/Annum
Production Capacity

Spread over a 60 acres with modern and global standard manufacturing units.
US FDA, WHO GMP Approved with
Award winning R&D center








Engaged in manufacturing high tech bulk drugs for international markets since 1984.

The company has prestigious USFDA approval & WHO GMP approval at its API plants

More than 100 satisfied customers world wide.

A wide range of different formulations e.g. tablets, capsules, powders, liquids, gels & ointments.

Strong documentation support by providing Technical Package of APIs, dossiers of the formulations.

Competitive global prices with quality product.